
We investigated the Brillouin zone and the electronic band structure of single walled carbon nanotube in the absence of perturbating.

The next two will go into the 2s orbital. (a) is the electronic band structure of chiral vector (6,0), (b) (6,3), (c) (8,0), (d) (5,5), (e) (8,8), (f) (5,4). Two of them will be found in the 1s orbital close to the nucleus. DFT functionals with a small amount of HartreeFock exchange fail to determine the experimentally observed polyyne molecular structure, revealing a cumulene-type geometry. The electronic band structures of several nanotubes according to (36) are illustrated. ** Except for helium, which has only two valence electrons. Cyclo18carbon (C18) is studied computationally at the density functional theory (DFT) and ab initio levels to obtain insight into its electronic structure, aromaticity, and adsorption properties on a NaCl surface. * The general method for counting valence electrons is generally not useful for transition metals. We also observe van Hove singularities at the onset of one-dimensional energy bands, confirming the strongly one-dimensional nature of conduction within nanotubes.\) Periodic table group

The bandgaps of both tube types are consistent with theoretical predictions. The diversity in electronic structure of carbon nanotubes arises from the quantinization of the electronic wave vector of the one-dimensional (1D) system. We observe bothmetallic and semiconducting carbon nanotubes and find thatthe electronic properties indeed depend sensitively on thewrapping angle. Electrons in shells Electrons in atoms occupy energy levels, also called electron shells, outside the nucleus. Here we present the results of scanning tunnelling microscopy and spectroscopy on individual single-walled nanotubes from which atomically resolved images allow us to examine electronic properties as afunction of tube diameter and wrapping angle.
Although the electronic properties of multi-walled and single-walled nanotubes have been probed experimentally, it has not yet been possible to relate these observations to the corresponding structure. In other words, similarly shaped molecules consisting of only one element (carbon) may have very different electronic behaviour. Carbon nanotubes can be thought of as graphitic sheets with a hexagonal lattice that have been wrapped up into a seamless cylinder. Solve any question of Classification Of Elements And Periodicity In Properties with:. You can see this more readily using the electrons-in-boxes notation. The modern structure shows that there are only 2 unpaired electrons to share with hydrogens, instead of the 4 which the simple view requires.

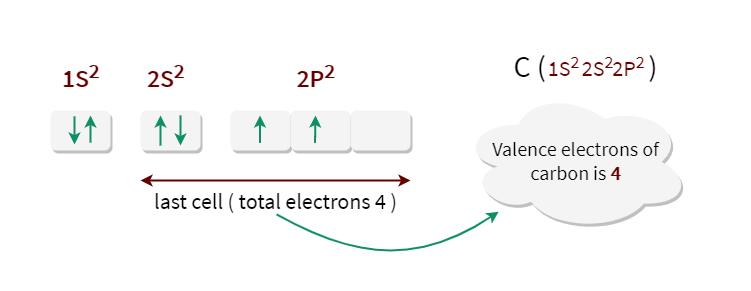
Their electronic conductivity, for example, has been predicted to depend sensitively on tube diameter and wrapping angle (a measure of the helicity of the tube lattice), with only slight differences in these parameters causing a shift from a metallic to a semiconducting state. the electronic configuration of Carbon is CZ61s 2 2s 2 2p 2. There is a serious mis-match between this structure and the modern electronic structure of carbon, 1s 2 2s 2 2p x 1 2p y 1. The valency of carbon is 4 as it has 4 electrons in its outermost shell and therefore needs 4 more electrons to complete its octet configuration. Since their discovery in 1991, the peculiar electronic properties of these structures have attracted much attention. Carbon nanotubes can be thought of as graphitic sheets with a hexagonal lattice that have been wrapped up into a seamless cylinder.


 0 kommentar(er)
0 kommentar(er)
